We’re here for your MBI servicing needs!
Universal Medical is teaming up with SmartBreastTM to provide servicing for Molecular Breast Imaging Products!
According to the American Cancer Society, there is a 1 in 8 chance that a woman will develop breast cancer at some point in her life. When it comes to detection in dense breasts,
mammograms have roughly a 30% chance of detecting early stages of breast cancer. However, SmartBreastTM MBI (Molecular Breast Imaging) systems raise that 30% rate of early detection up to 94%.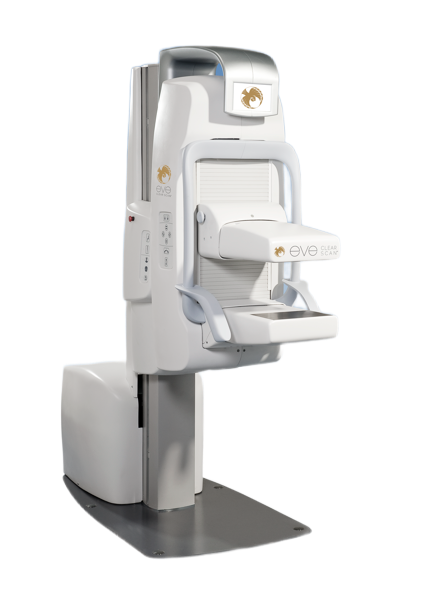
SmartBreastTM’s systems allow for women with dense breast tissue to have a better fighting chance against breast cancer. Earlier detection results in faster and more effective treatment.
Universal Medical will now be servicing two SmartBreastTM systems. The Eve ClearScan e680 and the Eve ClearScan e750, formerly the Dilon 6800 and GE Discovery NM750b, respectively, have been added to the Universal Medical product support catalog.
We are honored to partner with such a commendable leader in the Molecular Imaging
community.
Check out www.smartbreast.com for more information!

Latest Industry Update:
In March 2023, the US FDA issued a new breast cancer mandate including federal dense-breast notification with a deadline for compliance set for September 2024. Additionally, a bipartisan-supported legislation called “Find It Early Act” (HR3086) was introduced. This Act requires payors to cover supplemental screening costs for women with dense breasts.

